Sir William Grove created the first fuel cell in 1839. It wasn’t until 1950, however, that another British scientist, Sir Francis Bacon, demonstrated the fuel cell’s practical use in a fuel cell stack. It was the magic of chemistry created in this stacking of fuel cells that allowed them to win out over other competing power systems when NASA was looking to increase the duration of manned space flights. NASA even called their first fuel cells Bacon Cells in honor of Sir Francis Bacon. The fuel cell’s early success in NASA’s manned space flights allowed fuel cell technology to literally “take off” in the 1960’s. Many of the types of fuel cells that we have today were spawned out of the R&D done by NASA in the 1960s as variants of the Bacon Cell concept.
One of the fuel cell types developed out of the R&D done by NASA, the PEM fuel cell, continues to show great promise today for both NASA and many other manufacturers in a wide array of practical applications. Over fifty years after NASA started using fuel cells to help get man to the moon, fuel cell technology is currently advancing at a break-neck pace in 2016. We have finally reached a socially visible fruition point of real world and practical uses of fuel cells in our life time.
So how does a fuel cell work? Let’s look at the basics
Just like a battery, a fuel cell has: electrodes, an electrolyte, produces electricity, and has no moving parts. But, unlike a battery, a fuel cell does not store energy, it creates electricity as long as hydrogen & oxygen are supplied as fuel. Whereas a battery must be charged to produce electricity, a fuel cell must be supplied fuel. In the most common types of fuel cells being produced today, hydrogen flows to the anode of the fuel cell where upon contact the proton & electron separate. Only hydrogen’s positively charged protons travel through an electrolyte to the cathode. The electrons cannot pass through the electrolyte and are forced around the electrolyte to the cathode creating electrical current. The positively charged protons, negatively charged electrons, and oxygen all meet at the cathode to combine to make water vapor & heat as byproducts of this chemical reaction. In certain other types of fuel cells, ions travel from cathode to anode, but overall the premise of all fuel cells remains the same: electricity is generated through an electrochemical reaction.

By combining hydrogen and oxygen to make electricity, heat, & water through a chemical reaction, there is no combustion whatsoever. Because there is no combustion, harmful emissions are zero or tiny fractions as compared to competing technologies. This is a key attribute of what makes fuel cells so attractive to so many non-profit & for-profit entities around the world. When fuel cells use hydrocarbons like biogas for fuel, their CO2 emissions are still much lower than when hydrocarbons are conventionally burned because fuel cells have much higher efficiencies than combustion systems. When fuel cells use hydrogen for fuel, the only emission is water pure enough to drink.
There are five different basic types of fuel cells even though technically there are many more types. All 5 basic types of fuel cells operate with different electrolytes and operate at different temperatures. Each fuel cell type is well suited for specific markets. Let’s take a look at the 5 major types of fuel cells:
- Alkaline Fuel Cells (AFCs)
- Phosphoric Acid Fuel Cells (PAFCs)
- Proton Exchange Membrane Fuel Cells (PEMFCs)
- Solid Oxide Fuel Cells (SOFCs)
- Molten Carbonate Fuel Cells (MCFCs)
Alkaline Fuel Cells
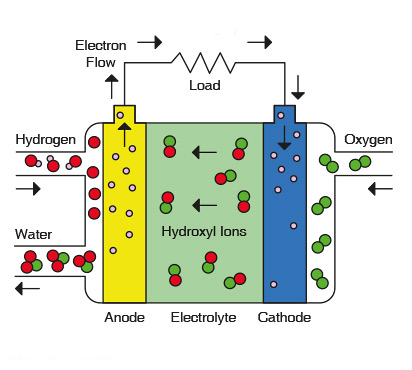 The most successful application of alkaline fuel cells has been in the US space program as mentioned in the opening paragraph. Alkaline fuel cells have supplied electricity to our US spacecraft and clean drinking water to our astronauts since the very first manned Apollo missions in the 1960s. The alkaline fuel cell uses only pure hydrogen and pure oxygen because of the AFC’s sensitivity to carbon dioxide contamination from using ambient air. The AFC electrolyte consists of a highly conductive potassium hydroxide (KOH). The electrodes of an AFC consist of crystallite structures with gold plated nickel mesh conductors.
The most successful application of alkaline fuel cells has been in the US space program as mentioned in the opening paragraph. Alkaline fuel cells have supplied electricity to our US spacecraft and clean drinking water to our astronauts since the very first manned Apollo missions in the 1960s. The alkaline fuel cell uses only pure hydrogen and pure oxygen because of the AFC’s sensitivity to carbon dioxide contamination from using ambient air. The AFC electrolyte consists of a highly conductive potassium hydroxide (KOH). The electrodes of an AFC consist of crystallite structures with gold plated nickel mesh conductors.
This paper written in 1989, gives an interesting look at the early history of the Bacon Cell that was the first commercial fuel cell used in our NASA space program. The Gemini V 1.0 kW acid IEM fuel cell (aka Bacon Cell) that debuted on August 1, 1965 was the advancement that gave our astronauts the electricity they needed to power longer manned space missions as well as clean drinking water. The Bacon Cell blazed the trail that has led to the advancements of fuel cells that we know today. All the advantages that fuel cells provided to NASA to make electricity and drinking water for our astronauts in outer space also have benefits making electricity and drinking water right here on earth.
Making clean energy and potable water from hydrogen fuel cells exemplifies two core pillars in RMP’s environmental mission: 1) to advocate for the production of clean & sustainable electricity with no harmful emissions, and 2) to advocate for conservation of Michigan’s & the world’s freshwater natural resources. The paper linked above also talks about genesis of the PEM fuel cell, which is the #1 type of fuel cell starting to dominate free-market applications. NASA has used fuel cells in every manned space mission since the fuel cell debuted, which is a testament to their success in the field. Now, in 2016, and 60 years after the first fuel cell went into space, technological advancements in fuel cells are happening at a faster speed than ever before.
NASA is currently looking more and more at the PEM fuel cell and plans to use long lived PEM fuel cells (10,000 hours) in future space missions to provide both electricity and clean drinking water for our astronauts. NASA talks here about how their alkaline fuel cells led to the PEM fuel cell and how their current research into PEM fuel cells will be key to supporting human life on the planet Mars. NASA’s experts at the Glenn, the Jet Propulsion Laboratory, the Johnson Space Center, and Kennedy Space Center are leading these current fuel cell research and development efforts. On NASA’s webpage here, they explain how they’re moving away from AFCs and moving toward PEMFCs & SOFCs which are fuel cell types explained later in this same article.
Phosphoric Acid Fuel Cells
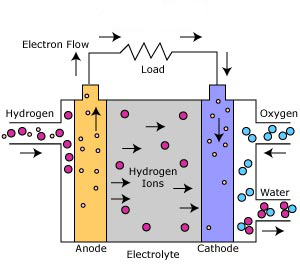 The Phosphoric Acid Fuel Cell is the most commercially developed of the fuel cells. It can operate using a variety of different reformed hydrocarbon fuels and uses ambient air as the oxidant. Phosphoric Acid Fuel Cells are already being used to generate electricity for hospitals, hotels, and office buildings.
The Phosphoric Acid Fuel Cell is the most commercially developed of the fuel cells. It can operate using a variety of different reformed hydrocarbon fuels and uses ambient air as the oxidant. Phosphoric Acid Fuel Cells are already being used to generate electricity for hospitals, hotels, and office buildings.
Phosphoric Acid Fuel Cells have the following characteristics:
- Operate at ~40% efficiency
- Operate at up to ~85% efficiency with co-generation
- Operate at ambient pressure
- Operate at low temperature (~400 F)
These four characteristics make PAFC fuel cells one of the most exciting types of fuel cells out there for stationary co-generation power applications. The last characteristic specifically, the low temperature, is a very intriguing one. Because PAFCs operate at a low temperature, around 400 F, they essentially operate at lower cost because the stack does not need replacement as often as compared to higher temperature fuel cells like molten carbonate and solid oxide fuel cells. Carbon oxides will “poison” most types of fuel cells by dirtying the electrodes and diminishing the fuel cell’s functional efficiency over time. An advantage of PAFCs is that they can tolerate a concentration of carbon oxide “poisoning” of about 1.5%.

The stack is the heart of any fuel cell regardless of type. If you follow RMP, you know our mantra: always follow the money. If PAFCs can operate at a lower costs, that means they will make customers happier by providing more value. Listen to Jeff Chung, the President & CEO of Doosan Fuel Cell explain why his company is focused on PAFCs in this video from September 10, 2015. Doosan Fuel Cell has extensive knowledge in all types of fuel cells. They are focusing on the PAFC because they think they can make this type of fuel cell deliver increased value creation for their customers because of lower operating costs. Always follow the money, it is what drives decisions in the real world and is the shortest route to the truth.
In 2013, CBS installed a Doosan PureCell Model 400 phosphoric acid fuel cell at their Los Angeles studio at 4024 Radford Avenue. This time-lapse video shows CBS removing their old power generators and installing a sustainable, reliable, clean, and low emissions PAFC for generating electricity and heat at upwards of 80% efficiency because of co-generation. Many companies operating large facilities in urban areas are switching to PAFCs because no other form of power generation can compete with a fuel cell’s power density; not solar, not wind, nor any other form of power generation. This is good news for America’s & the world’s most storm vulnerable cities. Hurricane Sandy in October of 2012 was a category 3 storm that hit New York City & New Jersey and cut power to many people in and around major population centers. Fuel cells and a distributed grid can help stop large outages caused by future storms similar to Sandy because fuel cell systems like the ones discussed here have no transmission lines; electrical power and heat are generated on site. The cost advantages and resiliency in life & death situations make fuel cells like PAFCs a very smart choice for America’s hospitals when the power has just gotta stay on 24/7. Even if fuel cells polluted our air & water like our current centralized power plants pollute, they would still make more sense economically than centralized power plants. Fuel cells, however, do not pollute and are therefore a win-win solution for energy production. Fuel cells provide clean energy on top of all their other fundamental economic advantages.
Proton Exchange Membrane Fuel Cells
 The PEM fuel cell is maybe one of the most exciting types of all the fuel cells. The PEMFC is the same type of fuel cell used in all the gorgeous cars pictured throughout this website like the Lexus in the featured image photo. As mentioned earlier in the post, the PEM fuel cell is where NASA, a leader in fuel cell development, is focusing their research to provide electricity and drinking water for our astronauts for a possible mission to mars. The PEM fuel cell, wouldn’t you know it, also has a variety of applications right here on earth that have many of us “in the fuel cell community” excited to see what will happen next. PEM fuel cells, for example, are powering Apple iPhones up to one week at a time with nearly zero wait to fully recharge again for another week. How cool is that?!? PEM fuel cells are scalable from tiny electric devices, to drones that can fly up to two hours (compared to battery drones that can only fly for a few minutes), to cars, trucks, busses, even bigger trucks, and even larger marine craft. The best parts about PEMFCs are their ability to operate continuously and their scalability. Lithium ion batteries are great and they’re a key component of most PEM fuel cell power systems, but as stand alone power systems, batteries are not even in the same ballpark when it comes to practical real world applications with real world markets. People want small cars, big cars, and even big trucks that can go 24/7, PEMFCs make this possible right now.
The PEM fuel cell is maybe one of the most exciting types of all the fuel cells. The PEMFC is the same type of fuel cell used in all the gorgeous cars pictured throughout this website like the Lexus in the featured image photo. As mentioned earlier in the post, the PEM fuel cell is where NASA, a leader in fuel cell development, is focusing their research to provide electricity and drinking water for our astronauts for a possible mission to mars. The PEM fuel cell, wouldn’t you know it, also has a variety of applications right here on earth that have many of us “in the fuel cell community” excited to see what will happen next. PEM fuel cells, for example, are powering Apple iPhones up to one week at a time with nearly zero wait to fully recharge again for another week. How cool is that?!? PEM fuel cells are scalable from tiny electric devices, to drones that can fly up to two hours (compared to battery drones that can only fly for a few minutes), to cars, trucks, busses, even bigger trucks, and even larger marine craft. The best parts about PEMFCs are their ability to operate continuously and their scalability. Lithium ion batteries are great and they’re a key component of most PEM fuel cell power systems, but as stand alone power systems, batteries are not even in the same ballpark when it comes to practical real world applications with real world markets. People want small cars, big cars, and even big trucks that can go 24/7, PEMFCs make this possible right now.
Here are some of the characteristics for PEM fuel cells that make them a smart choice for automotive & electronic device applications:
- Operate at low temperature (~200 F)
- High power density
- Quick Startup
- Continuous operation (about 3 to 5 minutes to refuel vehicle similar to gasoline)
The PEM fuel cell works best and achieves its best efficiency at higher pressures. Work is being done to broaden the PEM fuel cells operation under a variety of conditions. The PEM fuel cell differs from other fuel cells in that it uses a solid electrolyte similar to a sheet of plastic wrap that you would use in the kitchen to wrap your food before putting it in the refrigerator. The solid electrolyte helps make manufacturing PEM fuel cells much easier by creating a tight seal when the fuel cell stack is compressed together with regular nuts & bolts (PEMFCs construction is amazingly simple – stack it and screw it together and you’re done – watch for yourself). The solid electrolyte in a PEMFC also reduces corrosion and extends the fuel cell life.
Scientists predict that because of the ease of manufacturing of PEM fuel cells, their costs are expected to drop lower than the cost of Internal Combustion Engines (ICEs). The US Department of Energy is also predicting that the fully loaded “at the gate” cost of hydrogen to power PEM fuel cells will reach $2 GGE (Gasoline Gallon Equivalent).
Things are happening very fast with PEM fuel cells right now and their proliferation is just starting to hit a fever pitch. PEM fuel cell vehicles (like the ones shown throughout RMP’s website & twitter account) are production ready now and on the streets of many US states and other countries around the world.
There is no compromise in PEMFC vehicles as compared to gasoline or diesel-powered vehicles – no waiting, no issues with cold weather operation, no problem for big SUVs, trucks, & busses. The only thing currently holding them back from wide scale market adoption is the lack of refueling infrastructure which we can build to create good jobs and eliminate our need to import oil from hostile countries that support terrorism both directly and indirectly.

The PEM fuel cell shows great promise in automotive applications. Recently Hyundai and Toyota have introduced PEM fuel cell vehicles to the Southern California market. Later this year, Honda will sell their Clarity fuel cell vehicle in the Southern California market and become the third automaker to sell production model FCEVs. Hydrogen fueling infrastructure is only developed in Southern California but stations are springing up in many other states now too. The costs of building hydrogen fueling stations are coming down as each new station is built. Specifications and standardizations are starting to be adopted on a world scale and the proliferation of more hydrogen filling stations is rapidly increasing because of economies of scale.
RMP will be sure to tell you when the first public fueling station is built in Detroit. Detroit will most likely have a public hydrogen fueling station before the year 2020 but we can hasten their arrival if we decide we want a better economy sooner. Hydrogen refueling infrastructure is growing fastest in Southern California and RMP is advocating for Michigan to get into the game. State governments like those in California, Hawaii, Colorado, Connecticut, New York, and Ohio are advancing hydrogen infrastructure while Michigan’s private automakers like General Motors & Ford are working to advance fuel cell infrastructure here at home.
It’s time Michigan’s public sector got involved in advancing fuel cell adoption to help create sustainable jobs too. RMP has found a dataset that will allow for the mapping of all hydrogen fueling infrastructure across the country using our custom Google Maps API software. RMP can’t wait to see an #H2 fueling station with a Michigan latitude & longitude for one of our map markers. Follow us on Twitter or like us on Facebook to keep up with updates as things are happening fast.
Solid Oxide Fuel Cells
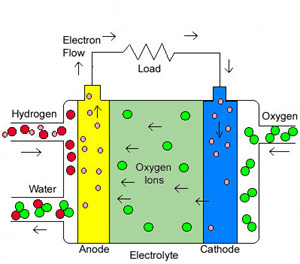 Both Solid Oxide Fuel Cells and Molten Carbonate Fuel Cells operate at much higher temperatures than other fuel cells, which means they can use a variety of hydrocarbons as fuel and operate at higher efficiencies than other fuel cells. By allowing for a variety of hydrocarbons for fuel, SOFC fuel cells are more simplified which can help reduce manufacturing costs. Solid Oxide Fuel Cells have the following characteristics:
Both Solid Oxide Fuel Cells and Molten Carbonate Fuel Cells operate at much higher temperatures than other fuel cells, which means they can use a variety of hydrocarbons as fuel and operate at higher efficiencies than other fuel cells. By allowing for a variety of hydrocarbons for fuel, SOFC fuel cells are more simplified which can help reduce manufacturing costs. Solid Oxide Fuel Cells have the following characteristics:
- Ceramic electrolyte
- Operate at High Temperature (~ 1,800 F)
- Operate at 45% efficiency
- Operate at up to 85% or more efficiency with co-generation
In a monolithic structured SOFC, each electrode is comprised of a corrugated layer (cathode) fused to two flat layers. A solid electrolyte is sandwiched between the corrugated cathode layer and a similar corrugated anode layer. Fuel flows through the corrugated channels of the anode while air flows through the corrugated channels of the cathode. An electrochemical reaction releases electrons, which begins the flow of electrical current. To

create a visualization of a SOFC in your mind’s eye, imagine the cardboard on a regular box. It has a flat layer on either side with a corrugated layer in between. Now imagine cutting multiple squares out of the cardboard and stacking them neatly one on top of the other like a Jenga tower. This is similar to how a SOFC monolithic structure would look where the pieces of cardboard in the stack would alternate anode and cathode. You can imagine looking at the profile view of this stack of cardboard pieces and seeing the airflow passageways that would allow for the flow of fuel & oxidant. In the case of SOFCs, the oxidant is just regular ambient air. Although it is slightly more difficult to fabricate the monolithic SOFC fuel cell structure configuration (like the cardboard analogy), it is projected to have a higher power density than other configurations like tubular or planar SOFCs.
Molten Carbonate Fuel Cells
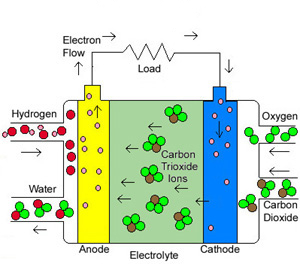 The MCFC is currently being tested for utility scale power generation. Like the SOFC, the MCFC promises to operate at high efficiencies and low emissions. The MCFC operates at high temperatures ( ~1,200 F). Because of the MCFCs high operating temperatures, they are also well suited for higher efficiency because of their co-generation capabilities. MCFC systems are ideal where both electricity and heat are needed on a continuous basis.
The MCFC is currently being tested for utility scale power generation. Like the SOFC, the MCFC promises to operate at high efficiencies and low emissions. The MCFC operates at high temperatures ( ~1,200 F). Because of the MCFCs high operating temperatures, they are also well suited for higher efficiency because of their co-generation capabilities. MCFC systems are ideal where both electricity and heat are needed on a continuous basis.
The electrolytes of MCFCs are made of potassium or lithium carbonates which when melted become ionically conductive. The MCFC stack structure is similar to the monolithic SOFC structure with corrugated flow channels for fuel & air. The difference between the SOFC and MCFC monolithic structure (i.e. the cardboard analogy) is that the corrugated channels are made with stainless steel while the electrodes themselves are made with porous nickel and nickel-oxide. Again, the higher temperature MCFC & SOFC fuel cells operation allows for the elimination of expensive platinum for a catalyst used in lower temperature fuel cells. This makes MCFCs inherently simple and potentially more cost effective than other fuel cells. As stated earlier, Doosan is betting that the PAFCs lower temperature will give their fuel cells better value creation because of longevity versus MCFCs higher efficiency.

A big advantage of MCFCs is that Carbon Dioxide, which is a considered a catalyst poison in other fuel cells, can actually be used directly in the MCFC providing additional fuel and further simplifying the system. The MCFCs ability to not be “poisoned” by CO2 but actually use CO2 as fuel has given Connecticut based Fuel Cell Energy a practical application to make great strides toward reducing CO2 and other polluting emissions from conventional power systems like coal burning plants and natural gas burning plants. On May 5, 2016 Exxon Mobil Corporation (NYSE: XOM) announced a partnership with Fuel Cell Energy (NASDAQ: FCEL) to explore using MCFC technology to effectively capture CO2 from the flue gas of natural gas burning power plants. This link provides information from ExxonMobil’s website. This is a link to a very informative one hour video with Vijay Swarup, the Vice President of R&D at ExxonMobil Research & Engineering Company and Chip Bottone, the CEO of Fuel Cell Energy. The location of the pilot plant being built by ExxonMobil & Fuel Cell Energy will be announced soon.
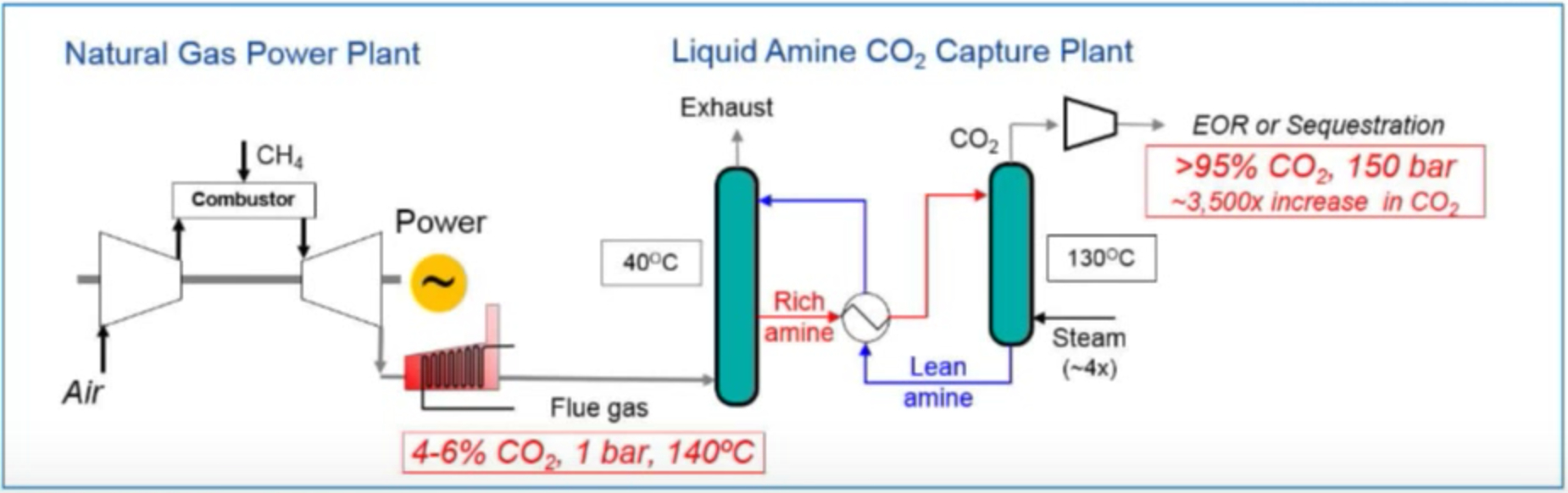
Below are a couple of diagrams prepared by ExxonMobil and Fuel Cell Energy that show the current generally accepted method of capturing CO2 using an amine system and Fuel Cell Energy’s new method of capturing CO2 using a molten carbonate fuel cell. The fundamental difference is this: the amine system requires energy and the fuel cell system provides energy. This is so fundamentally different and why so many research engineers are very excited to demonstrate this technology. It is a game changer. In the video link above, Vijay Swarup explains this fundamental difference at about the 26 minute mark. RMP will be blogging a lot more about this in future posts.
Amine Carbon Capture Plant
CO2 Capture System Using Molten Carbonate Fuel Cells
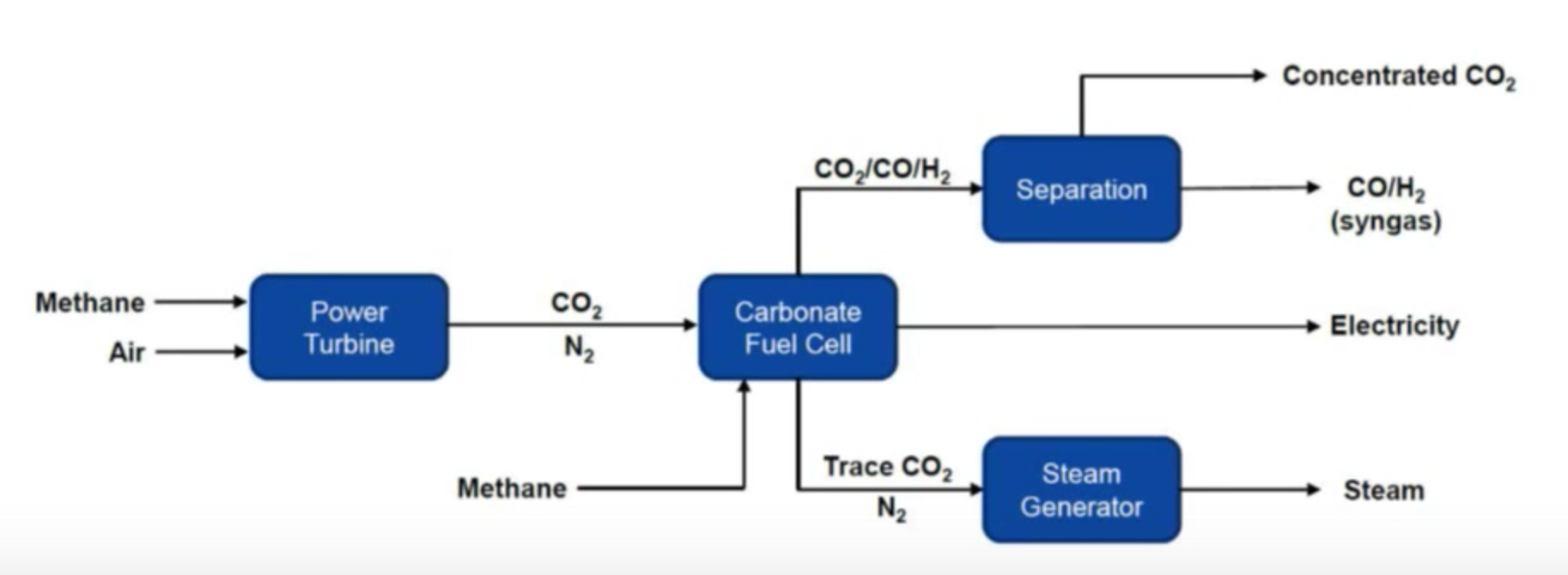
RMP is as excited about this technology earning its stripes in the free market. Soon ExxonMobil & Fuel Cell Energy will demonstrate its advantages to the world when they announce a pilot plant to show off this game changing technology. RMP could go on and on about this rapidly developing new method of carbon dioxide capturing but this post is just intended as an introduction to the 5 basic types of fuel cells. RMP will cover this story in detail as it unfolds in the coming months.
Just to add a little more color for our readers, below is an photo of what a molten carbonate fuel cell looks like on the production floor of Fuel Cell Energy which is located in Danbury, Connecticut. You can see its size relative to the men working on it to understand what it looks like. Depending on the size of the natural gas or coal fired power plant (i.e. 500 MW, 1000MW, 2000MW) these MCFCs can be added as needed which makes them very affordable versus amine CO2 capture. This is just another huge advantage of using MCFCs for CO2 capture.

Summary
So how good is this efficient & clean machine called the fuel cell? Whether for stationary operation or for transportation, the fuel cell far surpasses any other energy conversion device in its purity of operation. In transportation, even if a fuel cell bus uses a hydrocarbon like methanol for fuel, its emissions are only a small fraction of what they would be using an ICE; and with a fuel cell there are zero smog emissions and limited CO2 emissions. When a PEM style fuel cell operates to move a car or bus, using pure hydrogen for fuel, the emissions are ZERO and the only byproduct is pure water clean enough to drink.
In stationary applications when compared to internal combustion engines and turbines, fuel cells generate electricity at much higher efficiencies along with only a fraction of the emissions. Fuel cells are here today and more and more will become a part of our everyday lives tomorrow. It’s time to learn more about fuel cells to help accelerate their adoption not only for the good of our planet, but because they make so much economic sense as well. RMP is Michigan’s best fuel cell resource for staying on top of fuel cell news and technological advancements. Click here to like us on facebook and click here to follow us on Twitter.
Stay tuned to RMP to learn more about this exciting and fast developing technology that provides clean energy, helps our economy, and generates too many benefits to count toward protecting Michigan’s freshwater resources. Things are happening fast in the world of fuel cells and you can get all of your fuel cell news right here at this website.




Thank you for explaining how the fuel cell works for people that don’t know how it works. I like that it can be something that you can easily understand. How long do these cells work for? That might be something to find out.